A Global Warning for Millions of Diabetics
A major international safety alert has been issued after one of the world’s largest medical device makers confirmed that millions of diabetic glucose sensors may deliver dangerously inaccurate readings. The recall spans 17 countries, covers millions of devices, and is already linked to seven deaths and hundreds of serious medical incidents.
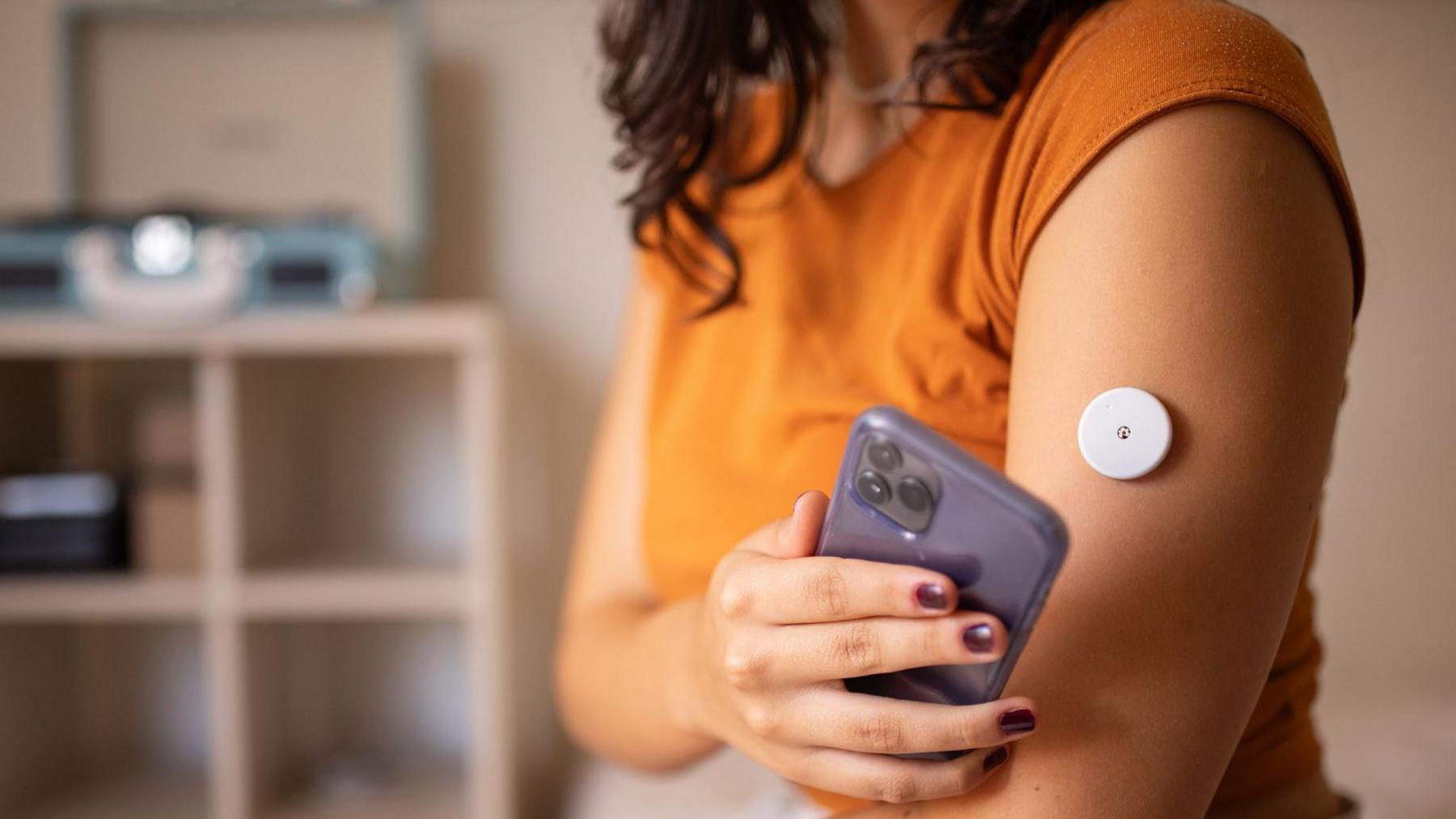
This week, healthcare company Abbott announced that specific batches of its FreeStyle Libre 3 and FreeStyle Libre 3 Plus glucose sensors were malfunctioning. These sensors are widely used by both Type 1 and Type 2 diabetics who rely on them to monitor blood sugar levels throughout the day. Accurate readings are essential for safe insulin dosing and for avoiding sudden highs or lows.
The issue came from a single production line, Abbott said, yet the impact spans the United States and sixteen other nations across Europe and beyond. In the U.S. alone, nearly three million faulty sensors may have been distributed. The scale of the recall has left doctors, patients, and caregivers scrambling for answers as health officials warn that a single incorrect reading can trigger a life-threatening crisis.
The Life-or-Death Risk of Faulty Readings
Diabetics depend on continuous glucose monitors to alert them when their blood sugar spikes or drops. A faulty reading can be catastrophic. If the sensor incorrectly reports a high number, the user may believe they are safe when they are actually approaching dangerously low glucose levels. This can lead to hypoglycemia, seizures, unconsciousness, or even death if untreated.
On the other hand, an inaccurately low reading may cause someone to take unnecessary insulin. Overcorrecting can send blood sugar plummeting with little warning. Experts emphasize that both scenarios can turn deadly in minutes, especially for people who live alone or rely heavily on their devices.
Abbott confirmed that the defective units tended to produce incorrectly low readings, which could lead users to either eat sugar they don’t need or delay necessary insulin doses. In both cases, glucose levels can swing into dangerous territory.
The company also stated that the recall does not reflect a broad failure of the product line, only a problem traced to a specific manufacturing process. Still, seven reported deaths and 736 serious health events, including 57 in the United States, have been tied to these inaccurate readings. For regulators, that number alone triggered immediate action.
Where the Recalled Sensors Were Distributed
The faulty devices reached customers in the U.S. and in the following countries:
Austria
Belgium
Canada
Denmark
Finland
France
Germany
Italy
Luxembourg
Netherlands
New Zealand
Norway
Spain
Sweden
Switzerland
United Kingdom
This global footprint means millions of diabetics must urgently check their device model numbers and stop using the affected sensors right away.
How the Sensors Work — and Why a Tiny Error Matters
Continuous glucose monitors use a small filament inserted just under the skin. This filament reacts to glucose in the interstitial fluid and sends a tiny electrical signal to the device. The system translates this signal into a digital glucose reading that appears on a smartphone or monitoring device.
A small flaw at any stage in this sequence can create a misleading number. And because many people check their glucose dozens of times per day, a faulty reading can influence eating patterns, insulin injections, and emergency responses.
Diabetes specialists stress that “just one inaccurate reading can begin a chain of decisions that puts the user in real danger.” These decisions stack quickly, raising the risk of diabetic ketoacidosis, severe hypoglycemia, or long-term organ stress.
Millions at Risk as Diabetes Rates Surge
In the United States, around 1.8 million people have Type 1 diabetes and more than 34 million have Type 2. Roughly 2.5 million depend on devices like the Libre 3 to track their glucose swings. With global diabetes cases expected to double by 2050, the reliance on accurate monitoring is only growing.
The new recall reignites debates about safety oversight in the booming medical-device industry. While Abbott insists it has “identified and resolved” the manufacturing issue, regulators and patient advocates say they expect more transparency about the exact nature of the defect.
What Patients Should Do Now
Abbott is urging all users to immediately stop using sensors with the following details:
FreeStyle Libre 3 sensors
Model numbers: 72081-01, 72080-01
UDI codes: 00357599818005, 00357599819002
FreeStyle Libre 3 Plus sensors
Model numbers: 78768-01, 78769-01
UDI codes: 00357599844011, 00357599843014
The company says replacement sensors will be available and that supply issues should remain minimal. Healthcare providers have begun contacting at-risk patients directly, but officials warn that many users may still be unaware their device is affected.
A Reminder of How Quickly Medical Technology Can Fail
Although modern monitoring systems have revolutionized diabetes care, this recall underscores the high stakes of even minor glitches. For millions of people, accurate glucose readings are not a convenience — they are a lifeline.
Health authorities say they will continue monitoring the situation as more information becomes available. For now, the immediate message is simple: check your device, stop using recalled sensors, and contact your provider for replacements.

Sarah Mitchell is a bestselling novelist recognized for her insightful and emotionally resonant stories that explore the complexities of human relationships. Originally from Denver, Colorado, Sarah grew up in a family of teachers who nurtured her curiosity and love for storytelling. She studied psychology at Stanford University, where she became fascinated by the intricacies of human behavior—an interest that would later shape her writing career. Sarah’s novels are praised for their nuanced characters, intricate plots, and ability to capture the subtle tensions that define love, friendship, and family ties. Her breakthrough novel, The Spaces Between Us, became an instant bestseller, lauded for its honest portrayal of strained family relationships and the fragile bonds that hold people together. Since then, she has published several works that continue to captivate audiences around the world. Outside of her writing career, Sarah is passionate about mental health advocacy and often partners with organizations to promote awareness and support for those struggling with emotional well-being. Her personal life is quieter—she enjoys hiking in the Colorado mountains, practicing yoga, and spending time with close friends. With each new book, Sarah Mitchell cements her reputation as a writer who illuminates the beauty and struggles of human connection.