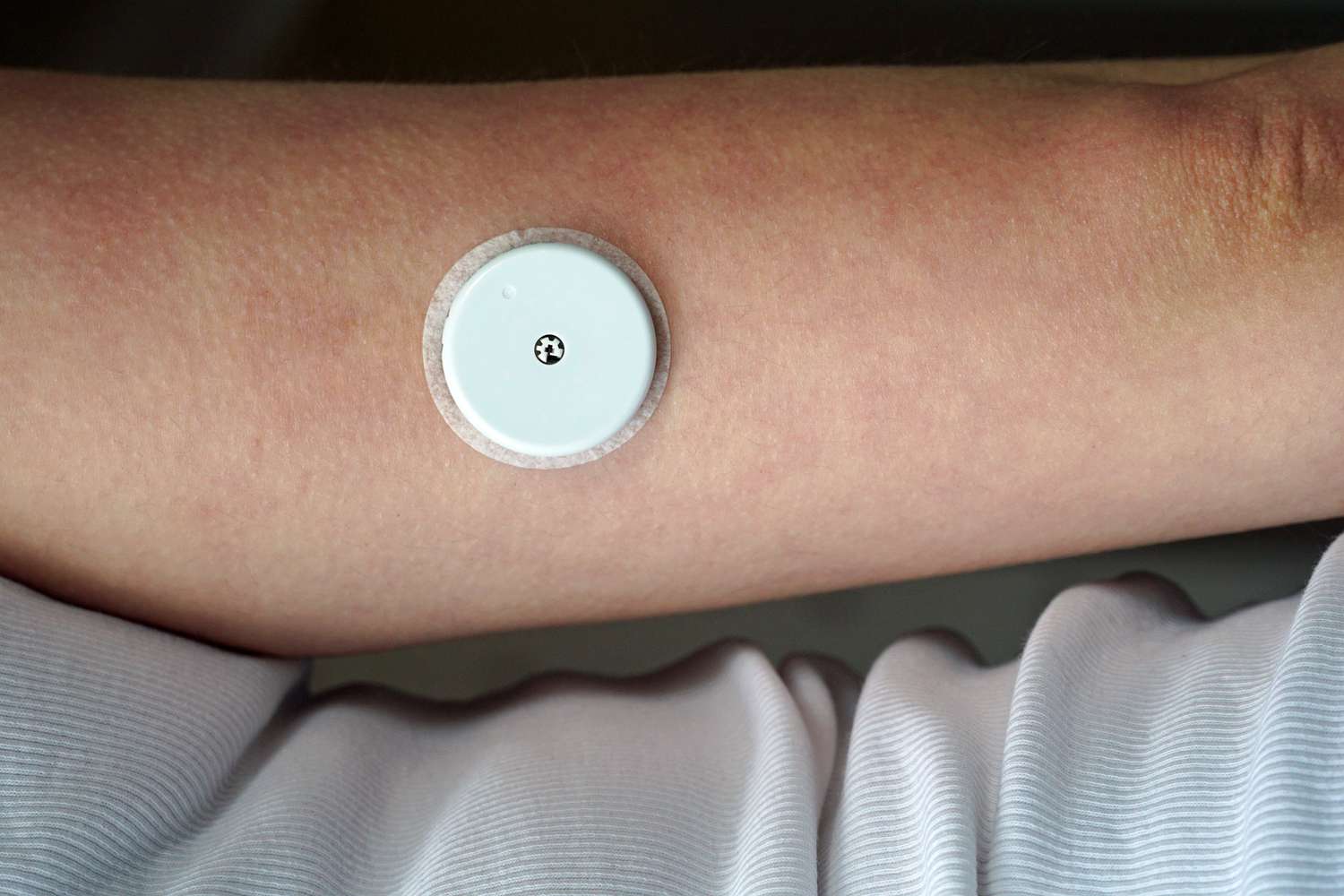
The U.S. Food and Drug Administration (FDA) has issued an urgent alert following reports of serious injuries and fatalities potentially linked to a widely used blood glucose monitoring device. Medical experts are urging patients to act immediately, as the problem could lead to dangerously inaccurate readings and severe health consequences.
The issue, identified through internal testing by the device manufacturer, involves readings that can falsely indicate low blood sugar levels. For people living with diabetes, relying on such inaccurate information can result in incorrect treatment decisions, ranging from overconsumption of carbohydrates to missed or delayed insulin doses. These errors carry serious risks, including injury or death.
Globally, seven deaths have been reported that may be associated with the malfunction. While none of these fatalities occurred within the United States, the incidents highlight the critical importance of ensuring that devices used for continuous glucose monitoring are reliable. In total, hundreds of adverse events have been reported worldwide, and more than 50 of these cases involved patients in the U.S.
Immediate Action Required
The FDA has emphasized that patients currently using the affected devices should not ignore this alert. Healthcare providers are also being advised to inform their patients of the potential dangers and to recommend alternative testing methods until the issue is resolved.
Patients are advised to closely monitor their blood sugar using standard glucose meters or backup methods. If readings from the device seem inconsistent with physical symptoms or prior expectations, individuals should not make any treatment adjustments solely based on those readings. Using multiple sources of information is critical to avoid complications.
What Went Wrong
According to the manufacturer, the problem was isolated to a specific production line among multiple lines that produce the devices. Sensors from this line may provide inaccurately low blood glucose readings, potentially misleading users into thinking their blood sugar is dangerously low when it is, in fact, normal or elevated.
This type of malfunction poses a particular hazard for people with diabetes who depend on continuous glucose monitoring for real-time decision-making. A false low reading could prompt unnecessary carbohydrate consumption, leading to hyperglycemia, or cause a patient to skip insulin doses, further endangering their health.
Which Devices Are Affected
Midway through the investigation, the company confirmed that the recall affects certain lots of the FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensors. Specifically, the affected models include:
-
FreeStyle Libre 3 Sensor: Model Numbers 72081-01 and 72080-01
-
FreeStyle Libre 3 Plus Sensor: Model Numbers 78768-01 and 78769-01
It is important to note that the readers and mobile apps associated with these devices are not affected, nor are other Libre models, such as FreeStyle Libre 14 day, Libre 2, Libre 2 Plus, or Libre Pro sensors. Abbott’s other medical devices are also unaffected.
Manufacturer Response
Abbott, the company behind these devices, stated that it has identified and resolved the root cause of the issue. The company is continuing to produce unaffected devices to fulfill new orders and provide replacements to customers with impacted units.
Consumers are encouraged to verify whether their device is part of the affected lots. Abbott has established a dedicated online portal, www.FreeStyleCheck.com, where users can confirm the status of their sensor by entering its serial number. Serial numbers can be found either within the device’s mobile app or directly on the reader.
For patients currently wearing an affected sensor, Abbott and the FDA strongly advise discontinuing its use immediately and switching to a standard glucose meter until a replacement is provided. The company has pledged to provide replacements at no cost.
Medical Guidance for Patients
Healthcare professionals emphasize that patients should never rely solely on a malfunctioning sensor. Cross-checking blood sugar levels with traditional meters is critical, particularly when sensor readings are inconsistent with physical symptoms or historical trends.
The FreeStyle Libre systems are designed to provide trend analysis and real-time data for managing diabetes, but they rely entirely on accurate sensor readings. When sensors fail, reverting to conventional testing methods is the safest approach.
Global Impact and Safety Concerns
The recall extends beyond the United States, with affected devices distributed in multiple countries. Although no deaths have been reported in the U.S., the global tally of seven potentially related fatalities underscores the seriousness of the malfunction. Hundreds of injury reports further highlight the need for immediate action.
The FDA alert is part of a broader effort to ensure public safety when medical devices pose a high-risk issue. By issuing an Early Alert, the agency aims to quickly inform both healthcare providers and consumers, minimizing the potential for further harm.
Patient Support and Resources
Abbott has expanded customer support to assist affected patients. Individuals can call 1-833-815-4273 for assistance, available daily from 8 a.m. to 8 p.m. ET. Live chat support is also available 24/7 through Abbott’s support website.
Patients can request replacements directly via the online portal once they confirm their device’s serial number. Healthcare providers are encouraged to guide patients through this process and advise on alternative glucose monitoring methods until a replacement is received.
The Importance of Accurate Monitoring
Continuous glucose monitoring has transformed diabetes care, offering users the ability to track blood sugar trends and make informed treatment decisions in real time. Devices like the FreeStyle Libre 3 and Libre 3 Plus improve patient outcomes by reducing reliance on finger-stick testing and allowing for timely intervention in response to changing glucose levels.
However, the reliability of such devices is paramount. Inaccurate readings undermine the very purpose of continuous monitoring and can have life-threatening consequences. Patients and providers alike must be vigilant, especially when discrepancies between readings and physical symptoms arise.
Looking Ahead
The FDA and Abbott have taken steps to limit the impact of the recall and prevent further incidents. Abbott continues to produce unaffected devices while addressing the batch-specific problem. Ongoing communication between the company, regulators, and patients aims to ensure safety and restore confidence in the technology.
This incident serves as a reminder of the critical importance of quality control in medical devices. Even a flaw affecting a small portion of production can have global consequences when millions of people rely on the product for daily health management.
Patients are encouraged to take immediate steps to verify their devices, follow replacement instructions, and continue monitoring their glucose using conventional methods when necessary. By doing so, they can reduce the risk of injury and maintain effective diabetes management while the recall is resolved.

Emily Johnson is a critically acclaimed essayist and novelist known for her thought-provoking works centered on feminism, women’s rights, and modern relationships. Born and raised in Portland, Oregon, Emily grew up with a deep love of books, often spending her afternoons at her local library. She went on to study literature and gender studies at UCLA, where she became deeply involved in activism and began publishing essays in campus journals. Her debut essay collection, Voices Unbound, struck a chord with readers nationwide for its fearless exploration of gender dynamics, identity, and the challenges faced by women in contemporary society. Emily later transitioned into fiction, writing novels that balance compelling storytelling with social commentary. Her protagonists are often strong, multidimensional women navigating love, ambition, and the struggles of everyday life, making her a favorite among readers who crave authentic, relatable narratives. Critics praise her ability to merge personal intimacy with universal themes. Off the page, Emily is an advocate for women in publishing, leading workshops that encourage young female writers to embrace their voices. She lives in Seattle with her partner and two rescue cats, where she continues to write, teach, and inspire a new generation of storytellers.